17 Jan 2025
Nutrition is a vital part of our nursing practice; there is significant evidence to show early enteral nutrition reduces hospitalisation times and improves survival rates for a range of clinical conditions.
We have to consider the best way to provide nutrition on a patient basis, and consider the complications associated with our chosen technique.
In this two-part article (Part 2 is here), I will explore the prevalence of under-nutrition within the veterinary field and the feeding options available to us to include oral feeding, tube feeding and parenteral nutrition. In our first part, I will discuss the different feeding tube options available.
The placement of feeding tubes is commonplace in practice, but this does not come without risk, and it is vital to consider how we can avoid these risks, and identify our patients with a higher risk of complications.
Naso-oesophageal (NO) tubes are often chosen, as they are quick to place and do not require an anaesthetic or sedation, although your patient does need to be quite amenable.
Camacho and Humm (2024) found one-third of animals required sedation for tube placement and two of these patients suffered major complications associated with this. The other consideration is that they will be much narrower than other tube options, so will require a thinner consistency food (Gajanayake, 2015), such as liquid feed, which comes in a range of types.
This thinner consistency food means a higher volume requirement, which may increase risk of regurgitation, and is more expensive as it is a special formulation. NO tubes are more commonly displaced than alternate tube options (Camacho and Humm, 2024), and the patient often requires a buster collar to prevent patient interference. There is also a risk of being placed into the bronchi instead of the oesophagus, which can have disastrous consequences (Oduyano et al, 2023). They are contraindicated in patients with protracted vomiting, oesophageal disease, poor gag reflex and altered mental status (Dumont et al, 2023).
Tube placement should always be preceded by five minutes with proxymetacaine or similar local anaesthetic into both nostrils and drops into each eye (Egleston, 2019). The eye drops allow local anaesthetic to travel down the back of the nasopharynx (McMurrough, 2021). This is an important step even if the patient is under general anaesthetic or sedation, as the patient will wake up and start aggressively sneezing, in my experience.
The tube should be measured to the ninth rib (Lumbis, 2017) to ensure it sits just above the cardiac sphincter – I mark this with a piece of tape, as watching for a pen mark or the number on the tube during insertion is challenging. Herring (2016) suggests also measuring the tube to the thoracic inlet and advancing to this point first, to prevent causing bronchopulmonary trauma from pushing the misplaced tube far into the airway, as there is a risk of causing pneumothorax by pushing the tube deep into the tracheobronchial tree. It is very rare for this to occur – 0.3% of patients – but these had a high mortality rate of 38% (Odunayo et al, 2023). Assessing the placement earlier can ensure correct positioning before advancing to the tube to the distal endpoint.
The tube is fed into the nostril ventromedially, and patients should start having a swallow reflex once the tube touches the back of the throat. If the patient starts coughing or gagging, I would be suspicious it is heading down the wrong way or coiling in the back of the throat, so I would remove and start again.
To check placement, I use a three-step rule.
None of the methods above correlated 100% with x-ray to confirm correct placement and, therefore, are not able to confirm tube position in 100% of cases. However, the stress to the patient and expense of x-ray needs to be considered.
Another method is doing neck and stomach ultrasound to confirm tube placement, which had a sensitivity of 98% and specificity of 50%; however, this means that 50% of cervical ultrasounds would fail to correctly confirm tube placement (Lawson and Tolliver, 2019). Camacho and Humm (2024) found oesophageal ultrasound was unreliable at confirming placement; however, Bruno et al (2023) found oesophageal ultrasound had excellent reliability and validity with confirming tube placement in cats when compared with radiography; therefore, it may be an appropriate method of confirmation alongside other methods.
Placement under general anaesthetic has a higher risk, as a patient may not cough in response to the tube being introduced into the airway. Therefore, another method that can be used is monitoring the cuff pressure of the endotracheal tube during enteral tube placement. Fuchs et al (2007) used this method on 30 patients and found that when the enteral tube was misplaced into the trachea, this increased the cuff pressure by an average of 28cm H2O, compared to placement into the oesophagus only causing a minimal change of 1cm H2O. Therefore, an increase of 10cm H2O of cuff pressure detected mispositioning with 100% sensitivity and specificity in this study.
The placement should be checked before each feed to confirm the tube has not be regurgitated or vomited between feeds, which could cause it to be displaced into the respiratory tract (National Institute for Health and Care Excellence, 2017).
Once you are happy the tube is in place, it should then be sutured or stapled in place. Previously, I used tissue glue; however, this was difficult when it came to removal, as the glue pulled the fur off with it, causing discomfort to the patient and being unsightly to the owner. I put a staple or suture as close to the nasal fold as possible (but not through the nose itself), to reduce the risk of self-removal – one over the nose and then coil the NO tube against the outside of the buster collar and tape (Figure 1).
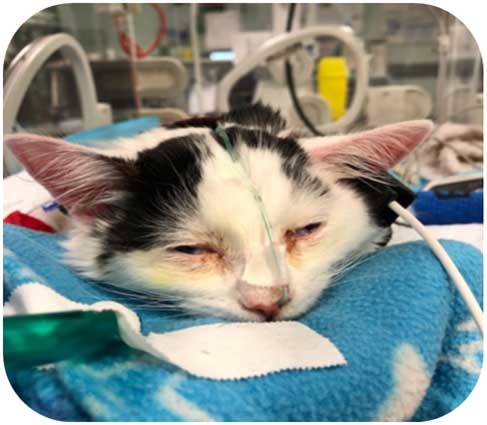
For nasogastric (NG) tubes, the same process is used as previously outlined; however, the tube is measured and placed up to the 13th rib. These are rarely placed in veterinary medicine due to the concern it increases gastric reflux through the cardiac sphincter, increasing oesophagitis risk.
However, these are the preferred in human medicine, as pH testing is done before every feed to confirm placement. It can also be used for gastric drainage or aspiration, as shown in Figure 2.
Camacho and Humm (2024) conducted a randomised controlled trial comparing complication rates between dogs and cats. They found that, out of a total of 82 dogs and 15 cats, the tube was misplaced in 3.1% of cases, all of which were dogs; this is similar to human studies, which report respiratory placement in between 1.3% and 2.4% of cases (Mardestein et al, 2004; Sorokin and Gottlieb, 2006).
The overall rate of complications during tube placement was 25.8%, with mostly minor complications reported. There was a slight difference in complication rates between NO and NG tubes, with NO having a 44.89% complication rate and NG having a 57% complication rate; however, this was not statistically significant. The most common complication in this study was sneezing (in 20 patients). There was no significant difference in regurgitation/vomiting rate or complications between the NO and NG groups.
Other common complications included vomiting up the tube, coughing, nasal discharge, regurgitation over 24 hours after placement, patient removal and mechanical obstruction. Yu et al (2013) had similar findings, with no difference in complication rate between NO and NG tubes in 46 dogs.
The other concern with the placement of NG tubes is that regular aspiration of gastric contents will cause the development of hypochloraemic metabolic alkalosis (HCMA). Chih et al (2018) aspirated the NG contents of 35 dogs and cats every 4 hours. This was compared to a control group that had no NG tube placement. Venous blood gas and electrolytes were checked every 12 hours. None of the patients developed HCMA. An increase in pH in the NG group was seen over time, but this was not statistically significant.
When testing placement for NG tubes, it is the same process as for NO, except you should aspirate a small volume of stomach fluid rather than getting negative pressure. However, Camacho and Humm (2024) found only 11 out of 48 NG tubes retrieved gastric contents. This fluid can be tested for pH, although this may be unsuitable for veterinary patients that are receiving proton pump inhibitors, as this study revealed a pH range of 5 to 9, and the animals with a gastric pH of 8 to 9 were receiving IV omeprazole (Camacho and Humm, 2024).
An additional test reported by Fan et al (2017) was auscultating the stomach during the water and air administration, which is used in human medicine. However, this is reported as an unreliable technique (Boeykens et al, 2014; Turgay and Khorshid, 2010), and this test did not correlate well with radiographs (Camacho and Humm, 2024).
Further methods that could be used include laryngoscopic-assisted placement and palpation of the NG tube in the stomach (Rodriguez-Diaz et al, 2021).
Tubes should be removed once patients are eating 80% of their resting energy requirement orally (Chan, 2020), although it is not uncommon for veterinary patients to self remove them. Due to the risk of tube displacement and danger of aspiration, it is not recommended for these tubes to be sent home in place.
Dumont et al (2023) monitored 119 cats and dogs sent home with NO tubes 24 hours after placement, and there were no major complications, although an overall complication rate of 65.5% was noted, with 18.9% requiring consultation. Despite this, owners reported a high level of satisfaction with using these.
Oesophageal (O) tubes are commonly placed for longer management of anorexia or inappetence. These can also be used in cases to bypass the oral cavity, such as jaw fractures or head trauma.
These can only be placed by a veterinary surgeon in this country and require a general anaesthetic. They allow for much thicker consistency foods to be provided, such as recovery diet or even a blended option of the patient’s normal food. Oral medications can also be crushed up and mixed with water to be given through this route.
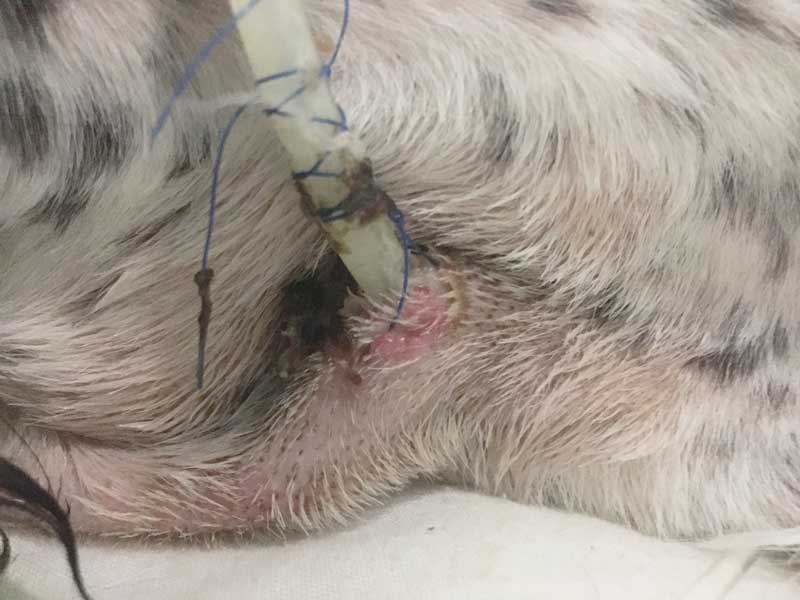
Higher risks are associated with this method – especially with infection around the insertion site (Figure 3) – but it has a much lower risk of aspiration or patient removal. Brunet et al (2022) conducted a study that included 112 cats with O (17%) or NO tubes (83%); 11% had a NO tube followed by an O tube. It was identified that complications occurred in 18.6% of cases, and these were similar with both tube types, with 15.3% having patient removal, 1% vomiting up the tube and 1% being placed into the airway in the NO group. Some 14.3% were removed by the patient and 14.3% of them had infection of the stoma site in the O tube group. The overall survival rate was 73%, with no significant difference between NO and O tube groups.
Return of appetite was found to be earlier in cats that had NO tubes placed (average of three days), while those with O tubes took an average of 33 days; however, this may be because cats with O tube placement were expected to require longer assisted feeding and, therefore, the decision was made for them to have an O tube placed. Hepatobiliary or upper respiratory tract disorders were more common in the O tube group, which are potentially more commonly long-term disorders. This compared to NO tubes, which were more commonly placed for digestive and upper urinary tract disease, which are more commonly short-term disorders.
Rare complications with O tube placement can occur, such as placement into the mediastinum (Kelly and Bandt, 2022).
A further alternative in addition to this is a pharyngeal tube, although this is technically more difficult to place. Rubini et al (2024) placed a pharyngeal tube in a four-month-old kitten with oral burns from benzalkonium chloride consumption; this was effective in avoiding the severe oral burns, allowing the oral cavity to heal while the patient maintained nutritional intake.
Gastric (G) tubes are placed to provide long-term enteral nutrition and bypass the oral cavity and oesophagus (Ao et al, 2015), and they will be used in cases of oesophageal foreign bodies and associated damage (Beer et al, 2022), severe oesophagitis and oesophageal strictures. It is unsuitable in patients with persistent vomiting, gastrointestinal obstruction or reduced consciousness (Marks, 2009). Gastrotomy tubes are placed in 43% to 60% of dogs undergoing oesophageal foreign body removal (Deroy et al, 2015; Sutton et al, 2016). Beer et al (2022) found gastrostomy tube placement was associated with reduced survival to discharge. However, this is potentially due to these being more likely to be placed in dogs with more severe oesophageal injury.
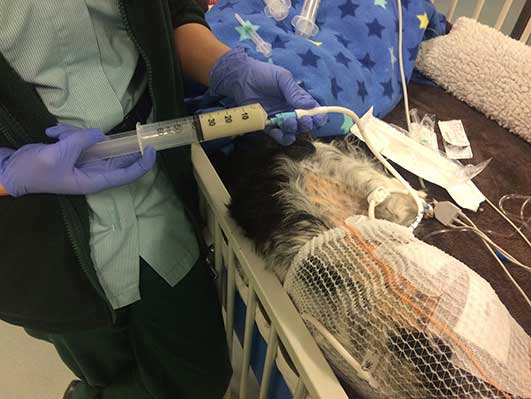
G tubes can be placed directly during abdominal surgery or placed endoscopically (PEG). It requires 24 hours’ post-placement to create a seal between the gastric wall and abdominal wall (Snyder, 2009), so can only be fed through after this 24-hour window (Figure 4). It also cannot be removed before 7 to 10 days, to ensure adequate adhesions have formed between the stomach and abdominal wall, preventing leakage of stomach contents and development of peritonitis. In immunocompromised or hypoproteinaemic patients, this may need be extended due to their reduced healing ability (Lumbis, 2017).
The benefit of the G tube is it allows for monitoring of gastric emptying and ileus, as prior to administering a feed the tube contents are aspirated (Figure 5). If more than 50% of the volume of the previous feed is aspirated, the next feed should not be given, and consideration should be taken of adding prokinetics such as metoclopramide or cisapride to increase gastric motility. This should then be reviewed at the next feed.

The gastric contents should always be replaced into the patient, to prevent causing a HCMA (Cook and Heinz, 2022; Figure 6). Increased gastric residual volume, vomiting and regurgitation are common complications not associated with the G tube itself; rather, they are common in our critically ill patients (Hansen et al, 2019). A G tube has a wide diameter, allowing for a wider selection of diets to be blended and fed through it.
Gastric tubes can present with minor complications in 26% to 33% of cases (Elmenhorst et al, 2020; Hansen et al, 2019), the most common being discharge around the tube site, which can cause uncomfortable acid burns – this occurs in approximately 23% to 66% of dogs (Elmenhorst et al, 2020; Hansen et al, 2019). Patients can also access these tubes, and self removal is a common complication (Elmenhorst et al, 2020; Hansen et al, 2019), but this can be managed with placement of a buster collar and securing the tube to the body using dressing retention stocking.
Reducing irritation around the site using barrier cream and/or surgical dressing (depending on level of exudate) is also recommended, as this will reduce the patients desire to itch or bite the area. Major complications can occur, such as the movement of the G tube out of the stomach into the subcutaneous space causing abscessation, requiring immediate tube removal (Hansen et al, 2019).
A gastric tube can also be managed at home by owners, making it a long-term solution for patients unable to eat orally. Owners need to be made aware of how to manage the stoma and checking for gastric emptying prior to feeding.
Jejunostomy tubes (J; Figure 7) are rarely used in veterinary practice, as the requirement to bypass the stomach is rare and has significant implications. The main reasons this may be placed is to reduce risk of aspiration from a vomiting patient, and reduce pancreatic stimulation in a case with severe pancreatitis. It is a technically difficult procedure and has a narrower lumen diameter, as well as requiring constant rate infusion feeding due to bypassing the stomach.
Ao et al (2015) compared the use of PEG tubes to J tubes within humans: J tubes had a much higher rate of complications, with 3 cases in 1,000 days requiring replacement, compared to 0.86 cases in 1,000 days with PEG tubes. The most common reasons for replacement were dislodgement or obstruction in the J tube group, whereas the reasons for replacement in the PEG group were most commonly routine replacement or dislodgement.
Hinden et al (2020) compared the use of a J tube with an O tube in dogs with severe acute kidney injury. They received food through the J tube for at least five days, followed by feeding through the O tube after removal; the control group was just fed through an O tube. There was little difference in complications between the two groups, although the J tube did take increased time to place compared to the O tube.
Three out of 10 of the J tube dogs also had oral displacement; however, there was no difference in metabolic improvement, despite the J tube requiring a specialised diet.