18 Jun 2025
Nutritional considerations – intervention and carer support
The clinical custom description
Nutrition is a vital part of our nursing practice. Significant evidence shows early enteral nutrition reduces hospitalisation times and improves survival rates for a range of clinical conditions. We have to consider the best way to provide nutrition on a patient basis, completing a nutritional assessment to provide a contextualised approach.
In this article (read part one on feeding tubes in VNT24.11), the author will explore the prevalence and risks of undernutrition and overfeeding, and consider the strategies to encourage oral feeding, contemplate the risks and benefits associated with continuous rate infusion versus bolus feeding, and lastly consider the correct use of parenteral nutrition.
Undernutrition in hospitalised humans has an estimated prevalence from 25% to 50% (Barker et al, 2011; Kelly et al, 2000; Lew et al, 2017), the prevalence of undernutrition in hospitalised cats and dogs varies from 25% to 65% (Brunetto et al, 2010; Remillard et al, 2001; Chandler and Gunn-Moore, 2004).
Molina et al (2018) found the average energy intake of 500 hospitalised dogs was only 23.9% of their resting energy requirement (RER), with 84% of dogs consuming less than 25% of their RER, and only 3.4% consumed their RER.
A total of 16% did lose weight and 18.4% hanta decline in body condition score (BCS) – especially older patients and those with a higher BCS on admit.
Death was associated with lower BCS and lower energy intake. Brunetto et al (2010) found energy intake has a positive association with discharge from hospital, and patients with a lower BCS had a lower hospital discharge rate compared to ideal or overweight BCS.
Those receiving voluntary food intake have the highest hospital discharge rate, with those receiving parenteral nutrition having the lowest. This may show a difference in survival to discharge due to severity of disease, as those receiving parenteral support were more likely to be critically ill than those that were voluntarily eating.
A more specific UK study looking at mechanically ventilated dogs identified only 31% received nutrition within 72 hours of no caloric intake (Greensmith and Chan, 2021).
Undernutrition in hospitalised patients is “stressed starvation” (Delaney, 2006; Gagne and Wakshlag, 2015), which is different to a healthy patient being starved for a procedure, “simple starvation”. Stressed starvation means the patient is in a hypermetabolic state (Robben et al, 1999) due to inflammation and sympathetic stimulation (Michel et al, 1997; Center et al, 2011), increasing energy loss and protein breakdown (Panel 1).
This in turn causes a negative energy and nitrogen balance (Robben et al, 1999). This loss of muscle affects immunity, wound healing and overall survival (Freitag et al, 2000; Baez et al, 2007).
Reduced food intake is important when considering quality of life in patients – especially those with chronic conditions such as congestive heart failure, cancer or chronic kidney disease. Reduced appetite occurs in 20.9% to 92.3%; however, this varies from anorexia to “reduced appetite” (Johnson and Freeman, 2017).
The lack of standardised terms for percentage intake can make nutritional assessment challenging. Therefore, the terminology stated in Panel 1 has been suggested by Johnson and Freeman (2017) to improve clarification of terms.
Early enteral nutrition
Early enteral nutrition is associated with an improvement in survival rates and reduced length of hospitalisation (Liu et al, 2012). This has been evidenced in critically ill patients with canine parvovirus (Mohr et al, 2003), haemorrhagic gastroenteritis (Will et al, 2005) and septic peritonitis (Liu et al, 2012).
Remillard et al (2001) found an association with a low-energy intake during hospitalisation and increased mortality rates. Critically ill patients have a high risk of malnutrition due to catabolism from the increased stress hormones and inflammatory mediators. They also are at high risk of hypoalbuminaemia, which is best managed through nutrition, although human albumin is available for severe cases (Mazzafero and Edwards, 2020).
The majority of these patients are inappetent or unable to eat due to a combination of factors such as nausea, ileus (commonly due to opioids) or reduced mentation and recumbency, putting them at increased risk of aspiration pneumonia (Remillard et al, 2001).
Jensen and Chan (2014) stated the importance of early enteral nutrition in humans with pancreatitis, although previously it was thought to be preferred to avoid the enteral route.
The same is now considered true in our dogs and cats, although a combination of contextualised approaches may be required. Therefore, nutritional support should be provided alongside antiemetics and prokinetics as deemed necessary, and the method chosen should be based on risk:benefit analysis. Voluntary food intake can also be encouraged through management of stress in the hospital environment and use of appetite stimulants (Dumont et al, 2023).
Some patients may be “pseudo-anorexic” where they are interested in food, but unable to eat orally – for example, jaw fractures or neurological issues causing absent gag (Taylor et al, 2022). In these cases, a proactive approach to nutrition, providing long-term sustenance, is required.
Nutritional assessment
Each patient is an individual, and we should be providing contextualised care to all patients according to their disease processes, temperament, breed, life stage, activity level, neuter status and owner wishes (National Research Council, 2006; Bermingham et al, 2010).
The nutritional assessment should be regarded as the fifth vital assessment, after temperature, pulse and respiration, and pain (Freeman, 2011) – especially identifying those at risk of malnutrition or that will require feeding intervention (Taylor et al, 2022).
Table 1 is a nutritional assessment table with a range of considerations for each patient when determining what assisted feeding route is required, if any (Chan, 2020).
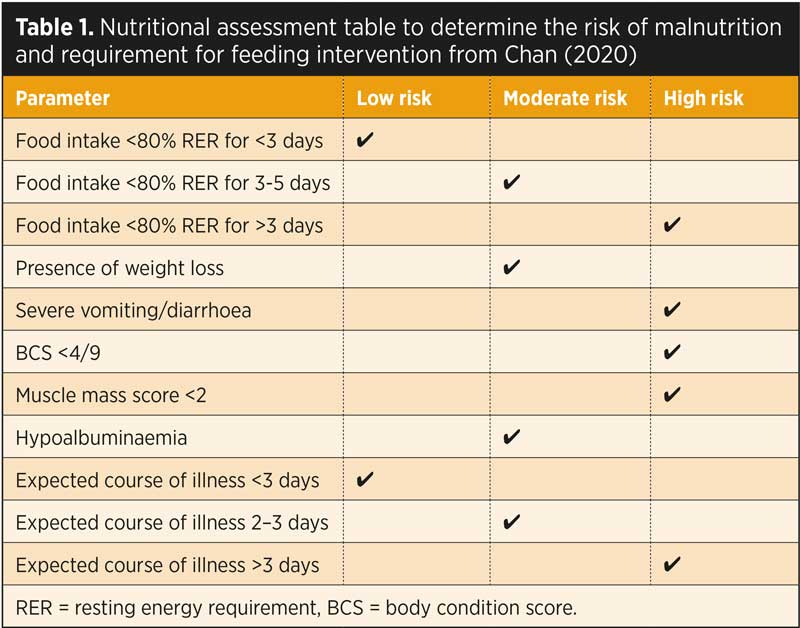
The nutritional assessment should include dietary history, clinical history, a physical examination that includes BCS/muscle condition score (MCS) and assessment of coat quality, assessment of any recent weight loss/gain and clinicopathological parameters such as electrolytes, albumin and creatine kinase (Chan, 2020).
Patients should be reassessed throughout hospitalisation, as the patients’ clinical status, appetite, BCS and MCS change (Michel, 2015).
Cats have a higher protein requirement than dogs, with the recommended protein intake for critically ill cats consisting of 60g/1,000kcal to 80g/1,000kcal (Eirmann and Michel, 2014; Chan, 2015). This is higher than the normal recommended intake due to increased protein losses that occur during illness.
Glutamine is an amino acid that provides energy for enterocytes and the provision of additional glutamine can improve protection in the intestinal mucosa. Additional fatty acids can improve immune function, and zinc is found to have a positive effect on wound healing and preventing proteolysis (Robben et al, 1999).
Cats also have a higher requirement of macro-nutrients such as retinol, arachidonic acid, vitamin D and taurine. While dogs also require these vitamins, they can metabolise and extract these more effectively from plant-based sources (Morris, 2002).
Cats also tend to eat frequent small amounts – especially those that hunt outdoors (Bradshaw, 1996). They are also crepuscular and therefore feeding at dawn and dusk may be more suitable (Bowen, 2018) and an important consideration with turning off or dimming lights of hospitalised patients at night to allow for natural behaviours.
Where possible, cats especially should be offered the same diet they are receiving at home, as neophobia is a common issue. Therefore, presenting cats with unfamiliar tastes or textures in a hospital environment may increase inappetence and food aversion (Zoran and Buffington, 2011). Therefore, sourcing a dietary history is important to continue feeding a familiar diet (Taylor et al, 2022).
In the author’s experience, we often jump to what we think will be the most appetising food for our cats, such as fish or chicken, or warmed wet food, but often cats will be used to a dry biscuit diet at home, and she often finds when this is offered it will stimulate some cats to start eating.
When planning the RER, the aim is to stabilise the nutritional status of the hospitalised patient, not to increase weight or body condition back to normal. Therefore, we approach this conservatively, with gradual reintroduction of food in those that have been anorexic for more than three days to prevent refeeding syndrome.
Reintroducing the food over three days, with 1/3 RER on the first day, followed by 2/3 day two and full RER day three, reduces the risk of re-feeding syndrome (Chan, 2020); however, in some patients that have been anorexic for longer periods, the reintroduction may need to be more conservative, occurring over several days (Cook et al, 2021).
RER calculation should use the following formula and be calculated according to current bodyweight, regardless of BCS:
RER = 70 × bodyweight (kg)0.75
There can be further adjustment to RER by a maximum of 10% every three to four days based on weight gain/loss, which should be assessed every day (Taylor et al, 2022).
Certain patients will have additional requirements to RER – for example, neonates, depending on age, should receive two to three × RER and patients with catabolic conditions such as burns or tetanus require additional calories (Birkbeck et al, 2020).
The number and size of feeds is also affected by the amount of food required by the patient. A high-calorie diet, but suitable to the patients’ specific requirements (for example, puppy, renal, hepatic, gastrointestinal [GI] and so on) should be chosen. For a cat, the maximum bolus per feed ranges from 5ml/kg to 15ml/kg (Taylor et al, 2022).
Newer specific liquid diets are becoming available (Kathrani and Parkes, 2022) that can be tube fed to treat IBD. Although this study only had a small sample of five, this diet effectively resolved anorexia in these patients, with some improvements in albumin, globulin and cholesterol levels, although these were not statistically significant.
For pancreatitis a difference in diet exists between cats and dogs, with dogs recommended to have a low-fat GI diet, whereas cats have improved response to hydrolysed diets (Cridge et al, 2024).
Refeeding syndrome
Refeeding syndrome can be avoided with conservative reintroduction of food after a prolonged period of starvation. Refeeding syndrome is found to happen in humans even after short periods of starvation (Mehler et al, 2010); however, it is more likely in cats that have been missing (and assumed starved) for more than three weeks (Cook et al, 2021).
Main signs include depression, coma, weakness, haemolytic anaemia, glycaemic deregulation and severe electrolyte derangements – especially hypokalemia and hypophosphatemia due to the sudden surge of insulin causing electrolyte uptake into the cells. Thiamine deficiency also exhibits similar signs, and is a risk in a starved or malnourished cat (Cook et al, 2021).
At-risk cats should be identified as those that have been missing, likely to have had no food intake and have significant loss of body condition. Electrolytes should be assessed and any derangements treated prior to starting feeding. For these patients to be gradually reaching the RER across 4 to 10 days; with a maximum 20% increase each day, electrolyte and/or thiamine supplementation may also be required (Chan, 2015).
Overfeeding
Overfeeding – even to underweight patients – can cause significant problems such as hyperglycaemia and GI stress; vomiting and diarrhoea (Ramsey, 2012). Overhydration may also occur – especially in patients receiving fluids and oral food/water, therefore monitoring for these signs as well as keeping a clear record of fluid ins and outs is important.
Signs of overhydration are increased skin turgor, tachypnoea, dyspnoea, pulmonary crackles and nasal discharge. This is especially a concern in patients with increased risk of overload, such as cardiac disease or anuric/oliguric renal disease patients (Taylor et al, 2022).
Bolus versus continuous feeding
Bolus feeding is preferred in stable patients as it simulates the normal feeding pattern the patient would have. Most patients will tolerate small, but frequent, meals; however, in humans, normotension is preferable when bolus enteral feeding. It is preferable to do constant rate infusion (CRI) feeding in hypotensive or borderline hypotensive patients, to prevent vasodilation within the GI tract worsening hypotension (Singer et al, 2019).
CRI feeding also reduces the rate of vomiting and patients reach caloric targets quicker compared to bolus feeding (Klaus et al, 2009). However, Campbell et al (2010) and Rado-Blozonva (2023) found no significant difference in GI complications between those fed continuously and those fed intermittently.
This study also compared the percentage of prescribed nutrition delivered (PPND) between the two methods. Those fed continuously received a slightly higher PPND (99%) compared to bolus fed (92.9%); however, this was not statistically significant. Patients receiving a CRI must be positioned upright with their head/neck extended to reduce the risk of regurgitation and aspiration (Taylor et al, 2022).
Parenteral nutrition
Enteral nutrition should always be considered first, as this has the most beneficial effect on gut barrier function, which could reduce the risk of bacterial or endotoxin translocation (Mohr et al, 2003). Some cases may be too high risk for enteral nutrition due to aspiration, or persistent vomiting/regurgitation despite anti-emetics; therefore, parental nutrition can be considered (Liu et al, 2012).
Ideally, a portion of the nutrition will still be provided enterally to still gain the benefits from enteral nutrition (Preiser et al, 2015). Adverse effects exist that are associated with parenteral nutrition, such as the risk of bacterial infection in an often already immunocompromised patient, and the requirement of a central line to provide total parenteral nutrition, which requires a general anaesthetic for placement, as well as being a more expensive option (Perea, 2012).
The most common disease process requiring parenteral nutrition is pancreatitis and the most common complication is hyperglycaemia, lipaemia and hyperbilirubinaemia (Queau et al, 2011; Chan et al, 2002).
Chronic kidney disease in dogs, hepatic lipidosis in cats and a longer duration of insufficient calorie intake prior to parenteral nutrition had a negative association with survival. Therefore, a proactive approach to nutrition as discussed earlier is vital to improve outcome.
Patients that had received both enteral and peripheral parenteral nutrition had higher survival rates than those just receiving peripheral parenteral nutrition. Positive survival factors included longer duration of parenteral nutrition, enteral feeding in cats and enteral feeding in dogs with respiratory disease (Chan, 2002).
Peripheral parenteral nutrition is also an option for patients that are unable to have a jugular catheter and require short-term nutritional support (for example, prior to placement of a longer-term feeding tube solution), or have additional requirements in addition to enteral feeding. The solutions are lower in osmolality, but this also means they are lower in energy and protein (Chandler et al, 2000); however, a high risk of infection remains with this method.
Appetite stimulants
Three appetite stimulants are licensed for veterinary use: mirtazapine, capromorelin and cyproheptadine. Appetite stimulants may be appropriate for patients showing interest in food, but not eating, or those that are eating, but not ingesting sufficient calories. They are not indicated in critically ill patients, or those with acute vomiting or nausea (Taylor et al, 2022).
Mirtazapine reduces nausea and vomiting, working similarly to ondansetron (de Boer, 1996), as well as being an appetite stimulant; however, some studies have found it did not increase return of appetite (Brunet et al, 2022).
It is available as a transdermal formulation, which makes it much easier to administer to an inappetent patient (Buhles et al, 2018). It has been found to significantly increase bodyweight, as well as having reduced the adverse effects due to slower absorption (Poole et al, 2019).
Mirtazapine at higher doses is associated with side effects such as hyperexcitability, vocalisation and tremors, due to serotonin syndrome (Ferguson et al, 2016; Quimby, et al, 2011).
A newer appetite stimulant called capromorelin is a ghrelin receptor agonist (Rhodes et al, 2017). A freedom of information summary (US Food and Drug Administration, 2020) found cats receiving capromorelin had significant weight gain; however, common adverse effects are vomiting, hypersalivation and lethargy (Wofford et al, 2018). Transient bradycardia and hypotension have been reported, making it unsuitable in hospitalised, and especially critically ill, cats (Taylor et al, 2022).
A further appetite stimulant is cyproheptadine; however, this is not licensed in cats or dogs, and its efficacy is anecdotal (Agnew and Korman, 2014).
Anti-emetics and pro-kinetics
As well as appetite stimulants, it is important to also relieve nausea and vomiting in these patients. The three main anti-emetics licensed for use in veterinary patients are maropitant, metoclopramide and ondansetron (Kenward et al, 2017). The first line anti-emetic is maropitant, which binds to neurokinin receptors in the brain to inhibit nausea centrally.
Ondansetron binds to 5HTP receptors in the brain, and is found to be the most effective emetic for managing chemotherapy-related nausea and vomiting (Kenward et al, 2017). Metoclopramide is a good option in patients that are suffering from delayed gastric emptying and ileus, which is common in our critically ill patients due to drug therapy, electrolyte abnormalities, pancreatitis and hepatic lipidosis (Yalcin and Keser, 2017).
Cisapride is also an effective prokinetic, stimulating gastric emptying and reducing oesophageal reflux – especially in cats – but is only available as an oral formulation, therefore its effectiveness may vary depending on gastric emptying (Whitehead et al, 2016).
Further considerations
Stress is a significant factor in the hospitalised cat, leading to inappetence (Mills, 2014). We need to ensure we are using canine and feline-friendly practices throughout our patients’ hospitalisation to minimise the detrimental effects of stress on the GI tract.
Regular pain assessment is paramount for administering sufficient analgesia, but not excessive, which may have adverse effects such as ileus and nausea (Gruen et al, 2022). Some patients may suffer more adverse effects than others from opioids. We need to consider our pain protocol on a patient basis, and observing any adverse effects, adjust accordingly.
Fluid deficits and electrolyte derangements should also be corrected as dehydration may cause constipation and inappetence (Taylor et al, 2022).
Hypokalaemia has a correlation with reduced appetite (Phillips and Polzin, 1998) and ileus (Husnik and Gaschen,2021) and is a common occurrence in critically ill and especially inappetent cats (Hoehne et al, 2019). Furthermore, supplementation with cobalamin in cats with B12 deficiency may also improve appetite (Simpson et al, 2001; Kempf et al, 2017).