23 Nov 2021
A case of canine atypical spinal choroid meningioma
Bartosz Ropelewski – referring to a case example – evaluates clinical symptoms, diagnostics and treatment options for this tumour type.

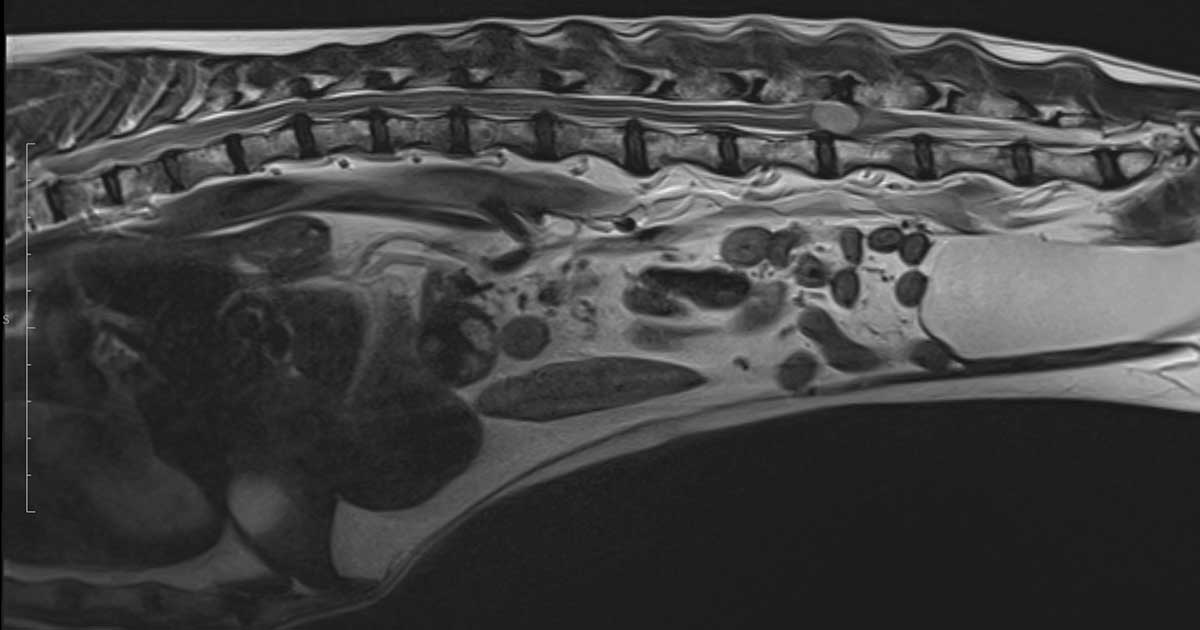
The T2-weighted sagittal scan shows high signal lesion at L4/L5 disc space.
A 13-year-old springer spaniel presented for investigation and treatment of a chronic spinal condition.
The patient had been reviewed by a first opinion vet 10 months earlier for investigation of pelvic limb issues. These included evident pelvic limb muscle weakness, proprioceptive positioning deficits and bilateral knuckling.
Initially, the dog was prescribed a long-term course of NSAIDs. The owner reported that during this treatment period, the dog’s condition became progressively worse.
At presentation, the dog displayed a number of neurological deficits including hindleg ataxia, bilateral proprioceptive deficit of the hindlegs, hindleg knuckling and panniculus deficit from the level of approximately the fourth lumber vertebra (L4).
Following discussion of possible treatment options, potential complications and prognosis with the owner, the patient was admitted for further investigation. An MRI scan was performed under general anaesthetic (premedication was with medetomidine 0.005mg/kg IV and methadone 0.3mg/kg IV; the patient was induced with 4mg/kg propofol to effect IV, intubated with a 7.5mm cuffed endotracheal tube; anaesthesia was maintained on sevoflurane and oxygen).
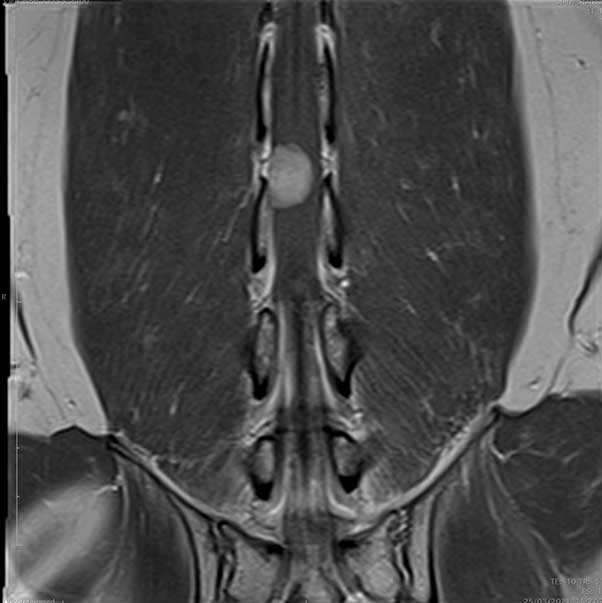
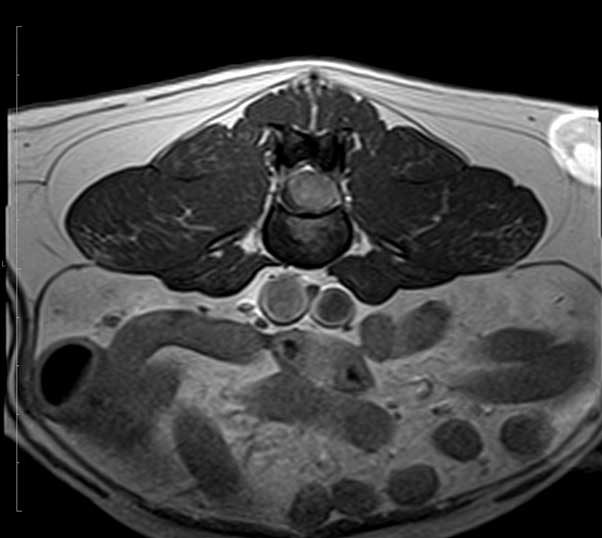
The MRI scan showed an intradural lesion, arising from the right side of the spinal cord and extending from the level of L4 to L5, to the mid-body of L5. The mass was an oval shape and measured approximately 1.26cm L × 0.7cm H × 0.95cm causing profuse spinal cord compression. The mass was homogeneous and hyperintense in T2W and isointense in T1W. No associated bone lesions were present.
After consultation with the owner, the decision was made to surgically debulk the spinal mass. Zinacef 15mg/kg IV and perfalgan 10mg/kg IV were given. Ketamine CRI throughout general anaesthetic was given at a rate of 0.6ml/hr reduced to 0.3ml/hr on recovery. IV fluids at the surgical rate 5ml/kg were given throughout surgery.
A dorsal midline incision was performed. The multifidus musculature was elevated with the use of the periosteal elevator. This exposure allowed sufficient elevation of the tendinous epaxial muscle attachments to help to visualise the lateral aspect of the spinous process, lamina, facet joint up to the level of accessory process. A right-sided L4 to L5 hemilaminectomy and durotomy was performed. The hemilaminectomy was performed using a surgical bur and Lempert rongeurs. The spinal nerve was identified and retracted with a blunt spinal hook, and the mass was debulked. The mass was submitted for histopathology.
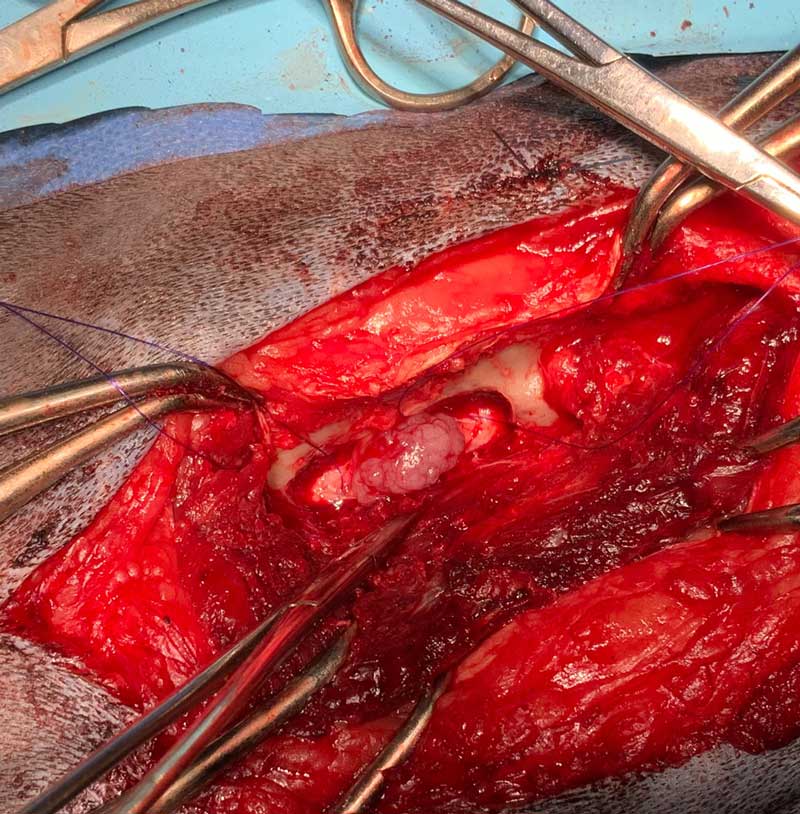
A urinary catheter was placed into the bladder after completing the surgical procedure. The patient recovered from anaesthesia uneventfully.
The patient underwent intensive physiotherapy during the period of hospitalisation. The urinary catheter was removed and spontaneous urination restored.
The dog gradually improved and was discharged to the owner. At discharge, the patient was walking with a sling support and had proprioceptive deficits on its right hindleg.
The dog was reassessed after four weeks; at this time it was walking without a sling support and had a very mild delay in proprioceptive positioning on its right hindleg.
Histopathology
The histopathology results showed that the mass had a multillobular structure.
Neoplastic tissue extended beyond the surgical margins. The lesion had moderate cellularity. The neoplastic cells were arranged in irregular nests and aggregated with short streams. Fibrovascular stroma supported the entire mass cellular structure. Most of the neoplastic cells were polygonal shape. A small amount of plump spindle shaped cells were present also.
Most of the neoplastic cells had a moderate amount of eosinophilic cytoplasm. A minority of the cells found in the lesion had amphophilic vacuolated cytoplasm. Nuclei had 0 to 2 nucleoli with stippled chromatin and were mostly oval in shape. There was less than 1 mitotic figure per 10 high power field with moderate anisocytosis and anisokaryosis.
The histological diagnosis confirmed a poorly differentiated neoplasm. Certain areas of the mass suggested meningothelial to transitional meningioma. However, other areas indicated that the neoplasm contained mucinous material, which can be found in chordomas. Further immunohistochemistry testing was performed, enabling further characterisation of the mass.
The immunohistochemical description showed that the tumour cells had moderate positive immunoreactivity for vimentin and strong immunoreactivity for glial fibrillary acidic protein (GFAP). Half of the cell population had weak immunoreactivity for S100. Cells’ immunoreactivity for cytokeratin and synaptophysin were negative.
Weak positive for S100 and strong for GFAP immunoreactivity could indicate that mass had a neuroepithelial, or neuroectodermal origin. In most cases, neuroectodermal masses have positive immunoreactivity for synaptophysin.
Localisation, history, clinical appearance and macroscopic structure of the mass strongly suggested spinal cord meningioma.
All the immunoreactivity results suggested that meningioma was unlikely as meningiomas tend to be negative for GFAP. However, cases have existed in the literature where positive GFAP expression was detected in meningioma (Barnhart et al, 2002).
Typically, immunoreactivity GFAP positive masses are nerve sheath tumours (Fetsch et al, 2005).
Chondrosarcomas are often GFAP positive, too. Immunohistochemical stain for osterix was evaluated and results were negative, therefore ruling out spinal chondrosarcoma.
The clinical features of the mass, in conjunction with histologic and immunohistochemistry findings, are most consistent with diagnosis of an atypical chordoid meningioma. However, it is impossible to completely rule out the possibility of peripheral nerve sheath tumour, although this type of mass would typically have a slightly different clinical pattern of growth.
Intraspinal meningiomas are identified as a primary nervous system tumour in dogs (Luttgen et al, 1980).
Meningiomas derive from meningothelial cells of the arachnoid membrane (Koestner and Higgins, 2002). Dog meningiomas have a lot of histological similarities to a human meningiomas (Sturges et al, 2008; Koestner et al, 1999). Therefore, the human World Health Organization (WHO) grading system is also used to classify primary nervous system tumours in dogs (Louis et al, 2007).
WHO grades I to III
Meningiomas are classified histologically according to the WHO into grades I to III. The three grades are:
- grade I (benign)
- grade II (atypical)
- grade III (anaplastic; Petersen at al, 2008)
The literature suggests that dogs’ meningiomas are localised mainly in the cervical and lumbar spinal segments (Gilmore, 1983; Levy et al, 1997; Beall et al, 2007). Low-grade I meningiomas can be found mainly in the cervical spinal cord and atypical grade II tumours are localised mainly in thoracolumbar spinal segments (Petersen et al, 2008).
In humans a strong correlation exists between prognosis and the grade of the tumour. The higher the grade of the mass the worse the clinical outcomes (Roux, 1996). Interestingly in dogs, the same pattern is not found (Petersen et al, 2008).
Presentation and clinical symptoms in animals with spinal cord meningiomas can be different depending on the duration and localisation of the mass. Early lesions may not manifest themselves for months and/or cause minimal clinical symptoms. Chronic masses can cause significant neurological deficits, due to the level of spinal cord compression.
Four spinal segments can be distinguished: C1 to C5, C6 to T2, T3 to L3 and L3 to S2. Clinical symptoms may present themselves in a different manner, depending on the localisation of the lesion.
For example, lesions affecting the proximal cervical spine will affect all limbs and initiate upper motor neuron signs. Masses localised at the level of C6 to T2 will affect all the limbs, too, but manifest as a combination of upper motor neuron signs in the back legs and lower upper motor neuron signs in clinical symptoms may present themselves in a different manner in front legs.
Compression at the level of T3 to L3 will cause hindleg deficits (upper motor neuron signs). Meningiomas present at the most distal spinal cord segment L3 to S2 will also affect the hindlegs, causing lower motor neurone symptoms.
In most cases, clinical presentation of spinal cord meningiomas tend to have a chronic and slowly progressive nature (Forterre et al, 2002; Auger et al, 1996).
The most typically reported clinical symptoms in dogs include ataxia, paresis, various degrees of spinal pain and, depending on the size and the location of the mass, lameness, and urinary and faecal incontinence (Petersen et al, 2008).
According to the literature, the average age of a dog at diagnosis of spinal meningioma is 9.1 years (Petersen et al, 2008).
Males are twice as likely to develop spinal meningioma than females, with a ratio of 2:1 (Fingeroth et al, 1987).
No strong evidence exists of breed predisposition to spinal meningiomas; however, the breed most affected by this type of mass is boxers (Forterre et al, 2002; Zaki et al, 1975).
Treatment options for canine spinal meningiomas consist of conservative management and surgical mass removal. Depending on the case, radiotherapy can be recommended post-surgery (Levy et al, 1997; Forterre et al, 2002).
The mean survival time for spinal meningioma removal varies from 4 to 47 months (Levy et al, 1997; Forterre et al, 2002).
The most important factors associated with tumour recurrence and survival are WHO grade and extent of surgical resection. A considerable number of meningiomas of WHO grade I can be cured by surgery; however, a considerable percentage reoccurs after complete resection. Therefore, long-term tumour control remains a significant challenge in the treatment of benign meningiomas.
Dogs affected by low and atypical grade II spinal meningiomas generally improve post-surgically and can become asymptomatic for months to years. Surgical removal of spinal cord meningiomas resulted in significant neurological improvement in (71%) of dogs. Radiotherapy combined with surgical management can lower percentage of potential recurrence (Petersen et al, 2008; Bell et al, 1992).
Grade III anaplastic malignant meningiomas have a very high risk of recurrence post-surgically (Kleihues et al, 2002; Perry et al, 1997).
The most important factors associated with tumour recurrence and survival are WHO grade and also extent of surgical resection.
Conclusions
The unique histopathological results in this study case make it interesting due to the fact the immunoreactivity results suggested that meningioma was unlikely as meningiomas tend to be negative for GFAP. However, this case was similar to a very few isolated cases in the literature where positive GFAP expression was detected in meningioma.
The patient had a severely compromised spinal cord due to the large mass (WHO grade II), causing severe neurological deficits. Following surgery, the dog has made a good recovery. In spite of the possibility of mass recurrence, radiotherapy was declined by the owner. The short-term recovery has been good, but the long-term prognosis remains uncertain.
