5 Nov 2024
Sarah Hewitt reviews latest thinking on a cause of parasitic bronchitis that is increasingly impacting adult cattle as well as youngstock.

Image: Iordanis Pallikaras / Adobe Stock

Image © Iordanis Pallikaras / Adobe Stock
Lungworm in cattle causes a parasitic bronchitis (“husk” or “hoose”) impacting both production and welfare. It can present in a variety of ways – from severe clinical infection resulting in mortalities, through sub-clinical infection causing losses in production, to carrier animal status where cattle can spread the parasite without being adversely affected.
Costs in growing cattle have been estimated to be between £50 to £100 per head, and in adult dairy cows around £3 per head per day in lost milk production (Control of Worms Sustainably, 2023).
Although typically a disease of youngstock, an increased number of cases in adult cattle are being reported. This could be due to changes in grazing management and increased housing of youngstock, increased use of long acting anthelmintics, reduction in use of vaccination as a management option, insufficient quarantine protocols and recent mild winters followed by warm and wet summers (Pass, 2022; Forbes, 2018a).
Lungworm management is often considered alongside other endoparasite management plans; the anthelmintic control options are the same (group one, two and three wormers) and similar risk-based approaches can be applied considering the risk of the animal (how much immunity they are likely to have) and the risk of the environment the animal is in (including pasture use history and climate).
Differences also need to be considered, though; most importantly, a vaccine is available for lungworm, which can help with ensuring adequate immunity is acquired and maintained (Bovilis Huskvac: MSD).
Lungworm outbreaks can also be less predictable than parasitic gastroenteritis with high infection levels developing quickly, making the option of a vaccine in the “control toolkit” even more important.
Cattle lungworm (Dictyocaulus viviparus) mainly affects cattle, but can be seen in other ruminants – for example, deer. The epidemiology is complex, with seasonal patterns varying year to year and farm to farm.
Although lungworm outbreaks tend to be less predictable than parasitic gastroenteritis or fluke, risk factors exist that can help determine how at risk of clinical disease a group of cattle is – typically herds in wetter western areas are likely to be more at risk, and high-risk times of the year tend to be late summer to early autumn, although it isn’t impossible to see outbreaks in housed animals (Figure 1).
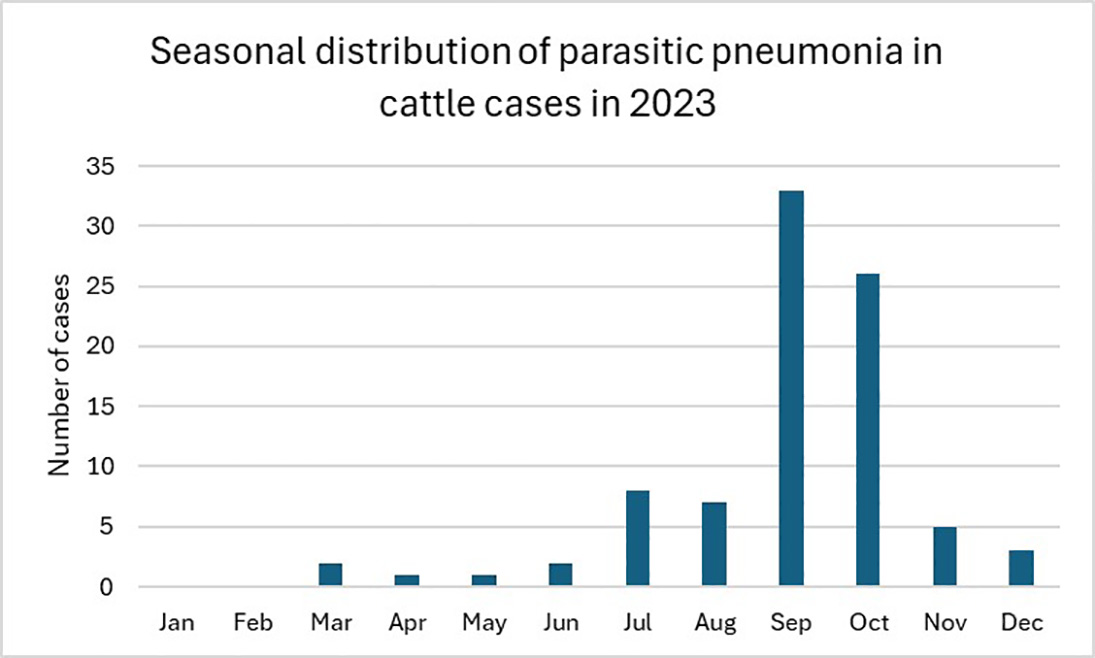
Figure 1. Parasitic pneumonia cases in cattle diagnosed by the APHA and Scotland’s Rural College during 2023.
Taken from VIDA annual report (APHA, 2024).
Immunity is generated on exposure to lungworm, so naive animals are most at risk of developing clinical disease. These are most commonly first-season grazers, but may also be older animals if they haven’t gained sufficient exposure in previous years (for example, if they have been brought in from a farm with no lungworm).
High-stocking densities also increase the likelihood that cattle will graze closer to pats and ingest infective larvae, increasing spread and risk of clinical disease.
In contrast to the parasites that cause parasitic gastroenteritis, lungworm is passed in the faeces in the larval L1 stage, rather than in an egg. L1 develop rapidly into L3 in the faeces.
This can take less than a week in warm and humid conditions, but may take several weeks when the weather is cooler. This influence of temperature on development rate means that lots of larvae may develop to the infective L3 stage simultaneously as it gets warmer over the summer, leading to high pasture contamination levels.
To facilitate ingestion of infective larvae by hosts, the parasite has help from a fungus. The Pilobolus fungus develops spores at the end of a stalk, which grow towards light (Figure 2). When the vesicle containing the spore bursts, spores can be catapulted up to three metres at speeds of up to 56 miles per hour (Petruzzello, 2018).

Figure 2. Pilobolus fungus. Image by Eduardo A. Esquivel Rios – This image is Image Number 236786 at Mushroom Observer, a source for mycological images (CC BY-SA 3.0)
Lungworm larvae climb the stalk to the vesicle containing the spore and are dispersed away from the dung pat at the same time.
When infective larvae are ingested, they become more active in the presence of bile. They cross the small intestinal wall and enter the lymphatic system, travelling to the mesenteric lymph glands, then to the thoracic duct and to the lungs. This is the “penetration phase” and commonly takes around a week.
They then colonise alveoli and bronchioles, ending up as adult worms at the base of the trachea or in major bronchi. This is the “pre-patent stage” and typically occurs 8 to 25 days post-infection. This promotes inflammation, resulting in eosinophil-rich mucus and parasite debris.
Irreversible epithelialisation of the alveoli exists, impairing cells’ gaseous exchange and leaving cattle more prone to other respiratory infections, contributing to the poor response to treatment sometimes observed. Adult worms produce eggs, which hatch into L1 larvae, are coughed up, swallowed and shed in faeces. This happens during the patent phase, around days 26 to 60 post-infection. The life cycle is outlined in Figure 3.
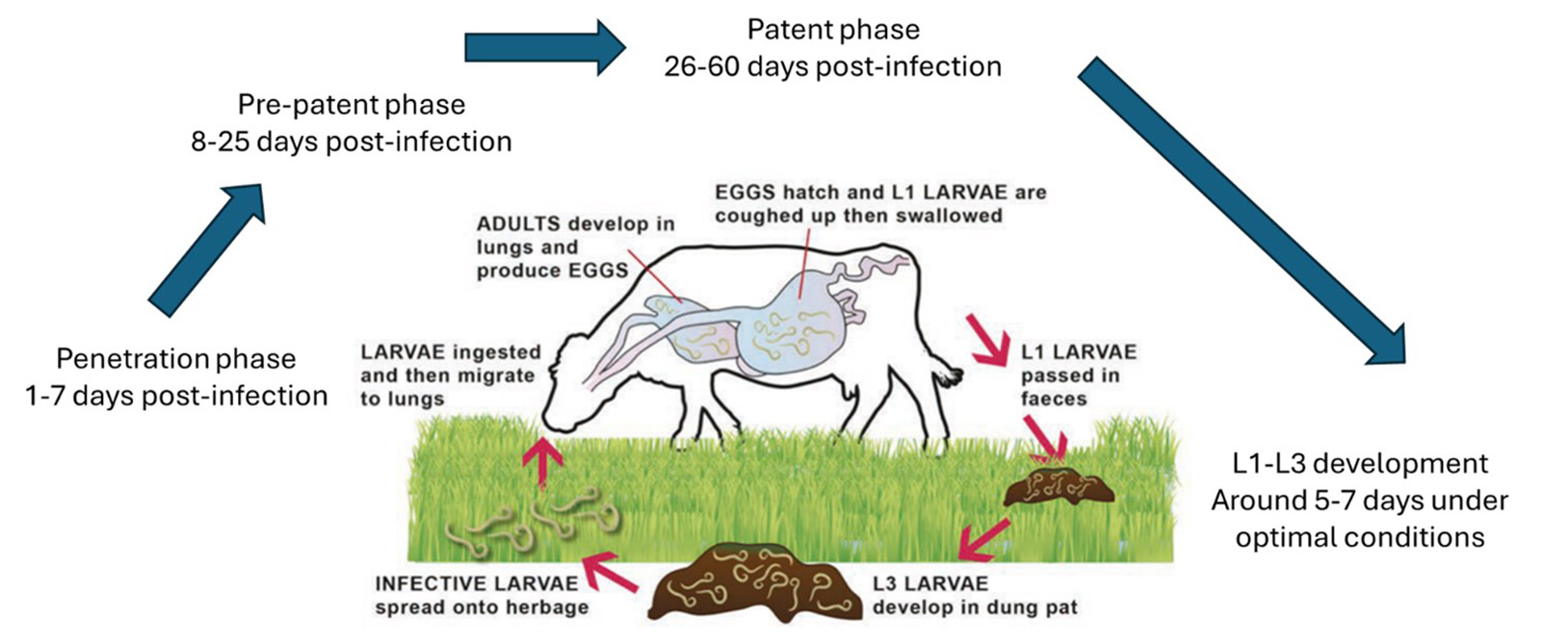
Figure 3. The lungworm life cycle (adapted from Control of Lungworm in Cattle, 2023).
The short pre-patent period of around three to four weeks, the fecundity of adult worms and the speed of L1 to L3 development in optimal conditions means that high infection levels can develop over a short period of time. Larvae can survive for more than a year on pasture if conditions are cold and wet.
Warm and dry conditions tend to lead to shorter larval survival times, as larval energy supplies are depleted more quickly when warm and dry conditions hinder dispersion from the dung pat. Larvae can also overwinter in carrier animals, which then contaminate pasture the following year. In some circumstances these animals are useful for providing low levels of contamination to boost immunity each grazing season (although this cannot be accurately managed).
Clinical presentation of lungworm can vary from acute mortality to clinically normal carrier animal status. Clinical disease commonly presents as coughing (this may be intermittent or frequent), increased respiratory rate and effort, and sometimes a mild pyrexia.
Milk drop is often seen in adult dairy cattle. Presenting signs are covered in more detail by Forbes (2018a).
Four phases of infection exist: the penetration phase, pre-patent and patent phases as already described, and a fourth post-patent phase. Post-patent parasitic bronchitis can cause death in apparently recovering animals a few weeks post-infection – typically around days 61 to 90. This is thought to be due to dissolution and aspiration of dead or dying parasite material.
Residual lesions may persist for some time after recovery, lung damage may be irreversible and the loss of ciliated epithelium can leave cattle more susceptible to other respiratory infections – all reducing the prognosis for a full recovery.
In addition to residual damage and post-patent disease, the possibility exists of re-infection syndrome if immunity wanes – for example, during the housing period. Immunity to D viviparus has two main components:
When cattle are not being exposed to lungworm, immunity can decrease. If this results in the first, shorter duration, immunity waning and cattle are subsequently turned out on to heavily contaminated pasture, large numbers of worms will reach the lungs.
Regarding the second component, longer duration immunity will kill large numbers of these mature worms in the lungs causing acute illness. This is termed “re-infection syndrome”.
A presumptive diagnosis is often made based on clinical signs and grazing history, along with the presence of risk factors such as season and climate. Additional diagnostic tests and their uses are summarised in Table 1.
| Table 1. Additional diagnostic tests and their uses | ||
|---|---|---|
| Diagnostic test | Lungworm stage detected | Uses |
| Baermann technique | Detects L1 larvae in faeces | Useful in patent infections, but may be negative in pre-patent or post-patent infections, and in re-infection syndrome |
| Broncho-alveolar lavage | Detects eggs, larvae and inflammatory cells in fluid flushed from lower airways | Can detect infections earlier than the Baermann technique, but may also be negative in pre-patent or post-patent infections and in re-infection syndrome |
| ELISA | Detects lungworm antibody to assess previous exposure on milk or serum | Can be useful for routine monitoring to look for changes in antibody levels. Antibodies persist for around six months after infection and so may indicate previous exposure rather than current infection. Seroconversion takes four to six weeks, so pre-patent animals will be seronegative and re-infected animals may also be seronegative |
| Postmortem | Detects adult worms in bronchi and bronchioles | Can be useful in cases of sudden death (with appropriate considerations for notifiable diseases) or where other testing or treatment has been unsuccessful |
All three classes of wormer licensed in cattle are active against lungworm, but oral drenches may cause more stress to administer than injectable or pour-on formulations, which is important to note for animals in respiratory distress. It is also worth noting that clinical signs may worsen following treatment in some animals.
When considering which wormer to use, duration of action is an important consideration if cattle are going back into a potentially “dirty” field. Macrocyclic lactones have persistent activity (four to six weeks), whereas levamisole and benzimidazoles do not. Supportive treatments (NSAIDs and possibly antibiotics) are also indicated in clinical disease.
Control strategies are commonly based on strategic anthelmintic treatment or vaccination. These strategies were initially developed for use in weaned calves from autumn/winter calving dairy herds and so can be more challenging to apply to spring or year-round calving dairy and beef herds (Forbes, 2018b).
Early season anthelmintic doses may be given to reduce pasture contamination, or rumen boluses may be used to provide sustained protection. With this approach the potential exists that insufficient exposure to lungworm during the first grazing season means that robust immunity to the parasite isn’t generated, although conflicting reports exist around this (Forbes, 2018a).
Anthelmintic resistance and environmental impact aspects also need to be considered. Targeted selective treatment strategies with the aim of reducing anthelmintic resistance and environmental impacts appear to be less useful in lungworm control compared to parasitic gastroenteritis control (Forbes, 2018b).
This strategy aims to keep cattle off high-risk pasture until any overwintered larvae on the pasture have died off (around April/May). This can be challenging practically, but considering if “clean” pasture exists for youngstock to go on early in the grazing season may also be beneficial (for example, pasture that didn’t have cattle on the previous year).
Grazing management strategies tend to be more difficult to apply to lungworm control compared to parasitic gastroenteritis as primary immunity is short lived, high infection levels can develop quickly and disease is less predictable. Recommendations for parasite control may also go against recommendations for grassland management (Forbes, 2018b).
Bolilis Huskvac can be given to cattle before their first grazing season and “boosted” pre-turnout in following years, but if low-level natural infection while grazing is present, this will also boost immunity (although this can be tricky to predict).
Vaccinated animals can become carriers, so it is important they are not mixed with naive animals.
A 2022 study investigated stakeholder perceptions of lungworm management and identified barriers to vaccine use (Pass, 2022). The cost of the vaccine (compared to the cost of wormer) and the requirement of two doses pre-turnout were highlighted challenges. The oral administration route and vaccine availability (it is a live vaccine produced during a short period and with a short shelf life) were also identified, as was a lack of communication from vets and SQPs about the benefits of vaccination.
The same study identified encouragement from milk processors to reduce anthelmintic treatments as a motivator for vaccine use. Anthelmintic resistance is beginning to be recognised as an issue in cattle (Bartley, 2011).
Whether this is a cause of increased lungworm diagnoses in adult cattle is unclear, but alongside the increasing awareness of the environmental effects of anthelmintic use (de Souza and Guimarães, 2022) and sustained public interest in how food is produced, vaccination is clearly an important tool in lungworm control.
Control of Worms Sustainably has launched a survey to collect information about lungworm outbreaks and treatment response during the 2024 grazing season. It is hoped that this will help to determine resistance levels and evaluate diagnostic and treatment approaches.
The survey can be found at www.cattleparasites.org.uk/cows-launches-lungworm-survey
Results will be released in 2025 and it is hoped they will help inform future strategy.