18 Jun 2024
Pros and cons of faecal egg count testing programmes
Jacqui Matthews weighs up the equine worming options for vets and clients.

Figure 3. Dung needs to be mixed for the best testing results.

Targeted worming programmes are now recommended for equine helminth control. This is due to the ever-increasing threat of drug resistance in these pathogens.
Anthelmintic resistance has been widely reported in cyathostomins and Parascaris species1, with emerging reports of reduced effectiveness of anti-cestode compounds against the tapeworm, Anoplocephala perfoliata2.
Each of these helminths has potential to cause serious disease, so sustainable control is imperative – especially as no novel compounds are coming to market in the foreseeable future.
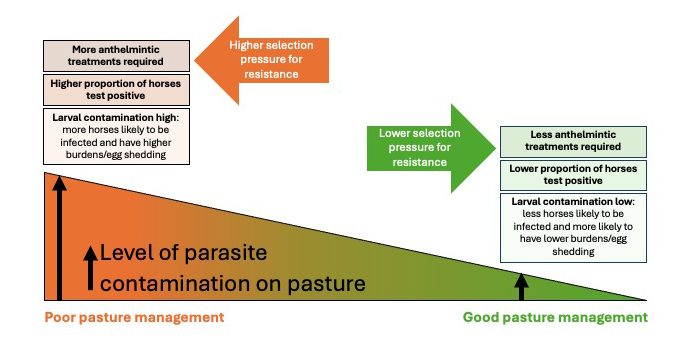
Procedures need to be applied that reduce our reliance on anthelmintics. These include environmental management of parasites and applying diagnostics that detect which horses require treatment. Management should focus on reducing levels of infective worm stages on pasture by breaking life cycles through regular (ideally, daily) dung removal, and maintaining good practice with respect to stocking density, pasture rotation/resting and quarantine.
Equine helminth diagnostic provision is now well-established in the UK, with faecal egg count (FEC) testing widely available through veterinary practices and specialist service providers. The past decade has brought innovative tests that measure parasite-specific antibodies that provide information on worm burdens3,4. As many horses have low helminth burdens/egg shedding profiles – particularly adult horses kept in low infection-risk situations (animals grazed on well-managed paddocks, or athletes with limited time at pasture) – the use of parasite testing can lead to considerable reductions in treatment frequency and, therefore, help protect efficacy.
Principles behind FEC testing
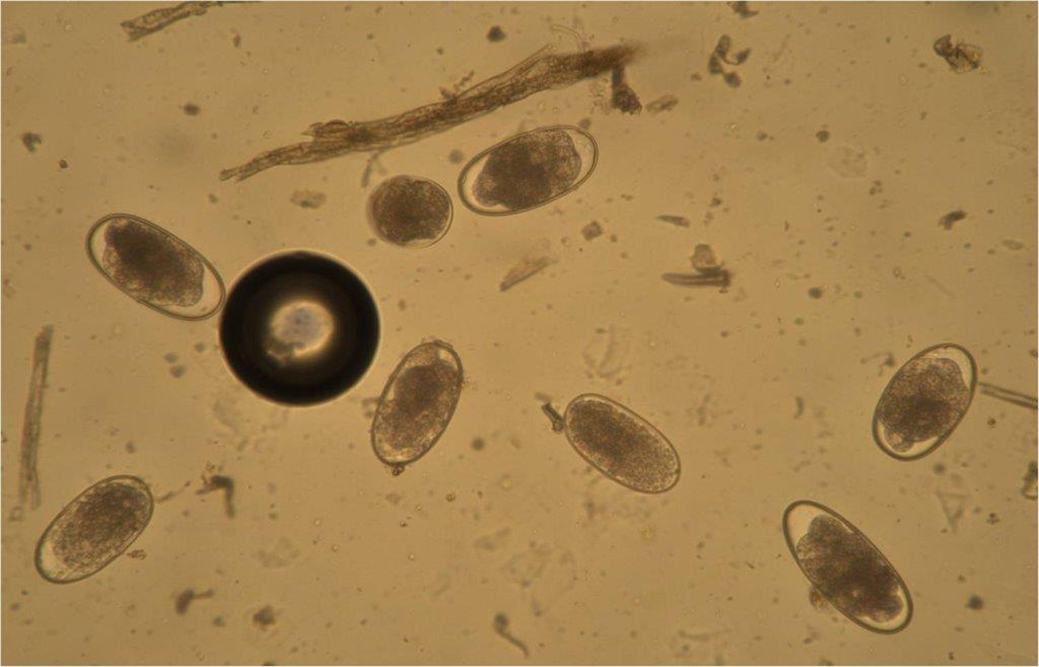
FEC testing is an essential component of control programmes – especially for cyathostomin infections, which are highly prevalent in animals of all ages, but with egg excretion higher in younger animals5. These tests are also invaluable for monitoring Parascaris species, a potentially serious pathogen of foals6.
Helminth egg shedding is over-dispersed in adult horses, meaning that, typically, more than 80% of eggs are excreted by less than 20% of individuals within a population7. Therefore, a relatively small proportion of each group is usually responsible for shedding the majority of eggs on to pasture. These patterns provide opportunity to reduce treatments by targeting horses above a specific eggs per gram (epg) threshold – often quoted as 200epg to 500epg8,9 – to reduce environmental contamination, while leaving a proportion of the worm population in “low egg shedders” untreated; therefore, reducing selection pressure for resistance10.
Although specific studies have not been undertaken in horses, evidence from ruminant nematodes suggests that such selective treatment strategies slow resistance by providing a pool of susceptible genes that dilute resistant alleles selected when treatments are given11. Studies demonstrate that, by using FEC tests, more than 75% treatment reductions can be achieved in horses7,12.
When paddock contamination is high, egg shedding is less over-dispersed and treatments will be applied to more horses based on FEC test results. In such circumstances, improvements in pasture management should be applied to lower pasture contamination to reduce burdens and, subsequently, worm egg shedding levels.
The relationship between management, diagnostics, treatment frequency and anthelmintic resistance selection is summarised in Figure 1.
In the UK, FEC testing should be performed in all horses at 8-12 weekly intervals throughout the grazing period (spring to early autumn). Test also at the end of winter if horses graze for significant periods year-round.
Timing of testing is dependent on the previous anthelmintic used; for example, test 8 weeks after treatment with fenbendazole, pyrantel embonate and ivermectin, and 12 weeks after moxidectin treatment. These timings can be shortened where reduced worm egg reappearance periods post-treatment have been identified, or in youngsters (less than five years). Within individuals, strongyle egg excretion levels are relatively consistent13,14, but can vary with time of year, with higher FEC more likely in spring/summer5.
Shedding levels are less consistent in youngsters15, probably because of their lower immunity, with increased larval challenge less affected by immune responses in the intestine.
Pros of FEC testing
FEC testing provides estimations of egg excretion levels of most common nematode species, and is relatively simple and inexpensive to apply.
The test provides the capability to monitor nematode egg contamination on to pasture and can be employed to achieve significant reductions in anthelmintic usage. It is non-invasive, and the methods developed for counting eggs are well validated and, when samples are properly handled, transferred and processed, it is an invaluable tool in control programmes.
It has high specificity for identifying strongyle eggs, but cannot distinguish large from small strongyle eggs, with larval culture required to discriminate these types of worms. It has high specificity for identifying other nematode species’ eggs, such as Parascaris species and Strongyloides westeri.
A key application of FEC testing is in the assessment of anthelmintic efficacy by applying the method in the FEC reduction test (FECRT). Because resistance is so widespread in cyathostomins and Parascaris species, efficacy testing should be an annual component of all control programmes. The FECRT calculates the percentage mean reduction in FEC 14 days after anthelmintic administration and provides important information on how well anthelmintic compounds are working on individual premises.
FECRT can be affected by various factors that affect the accuracy and sensitivity of FEC methods; these are discussed in the following section.
How to get the best value out of FEC analysis
FEC tests are sensitive to factors that introduce variability; these include how samples are collected and transported, and which method is used to count eggs.
As nematode eggs hatch quickly in air in warm conditions (above 20°C, hatching occurs in a day16), samples should be collected fresh or within 12 hours of being deposited.
As hatching is an aerobic process, samples should be placed in zip-lock bags with all air expelled or, if solid containers are used, these should be packed so that the sample fills the container. Ideally, faeces should be kept below 6°C, as hatching does not occur under this temperature, and samples can be stored at 6°C for up to 5 days before strongyle egg levels decline16.
Nematode eggs (Figure 2) are unevenly distributed in faeces, so a representative sample must be collected17,18. If only a small amount of material is collected from a single area of a pile, inaccurate results will be obtained15. At least 5g of material should be collected from a minimum of three faecal balls and samples must be thoroughly mixed before any sub-samples are taken for analysis (Figure 3).
The modified McMaster method19 is the most common technique used for FEC analysis in the UK. It uses a flotation-dilution principle to assess counts and assumes that eggs are randomly distributed in the counting solution. Therefore, samples must be well mixed just before placing on to the counting slide. If not mixed, and only the surface layer is removed, more eggs are transferred, leading to overestimations in FEC.
The sample volume assessed is also important – particularly for efficacy assessment. This is because when small volumes are examined, the method uses larger multiplication factors to achieve the epg estimate; this can inflate variance, leading to more variable results20. A higher number of false negative results will also occur when low volumes are assessed.
More accurate results will be obtained using methods that examine larger volumes of solution and have lower multiplication factors to calculate the epg result.
Cons of FEC tests
FEC tests do not provide an accurate measure of the total nematode burden within individuals, as they do not take account of adult male worms or larval stages. Furthermore, standard FEC methods do not reliably detect pinworm, lungworm, trematode or cestode infections. For these reasons, FEC tests cannot be used to provide accurate information about the involvement of specific worm types in clinical cases, nor can they be used directly to predict disease risk.
In the case of A perfoliata infections, studies that assessed FEC methods against the gold standard of total tapeworm counts postmortem demonstrated that, although specificity is generally high, sensitivity of coprological testing is low21. One reason for this poor sensitivity is that egg-containing proglottid release from adult worms is intermittent, leading to uneven egg excretion over time22.
Another confounding factor is that immature or sterile adult worms (neither detected by a test that measures egg numbers in faeces) can comprise a substantial proportion of the burden. For example, one postmortem study that counted different tapeworm stages in horses found that only 16% of the worms counted were egg-containing23. For these reasons, it is likely that A perfoliata prevalence will be underestimated by FEC testing.
Antibody-based tests provide a more sensitive option for detecting tapeworm infections. These are available in blood and saliva formats (Figure 4), with antibody levels measured shown to correlate with tapeworm levels3,24.
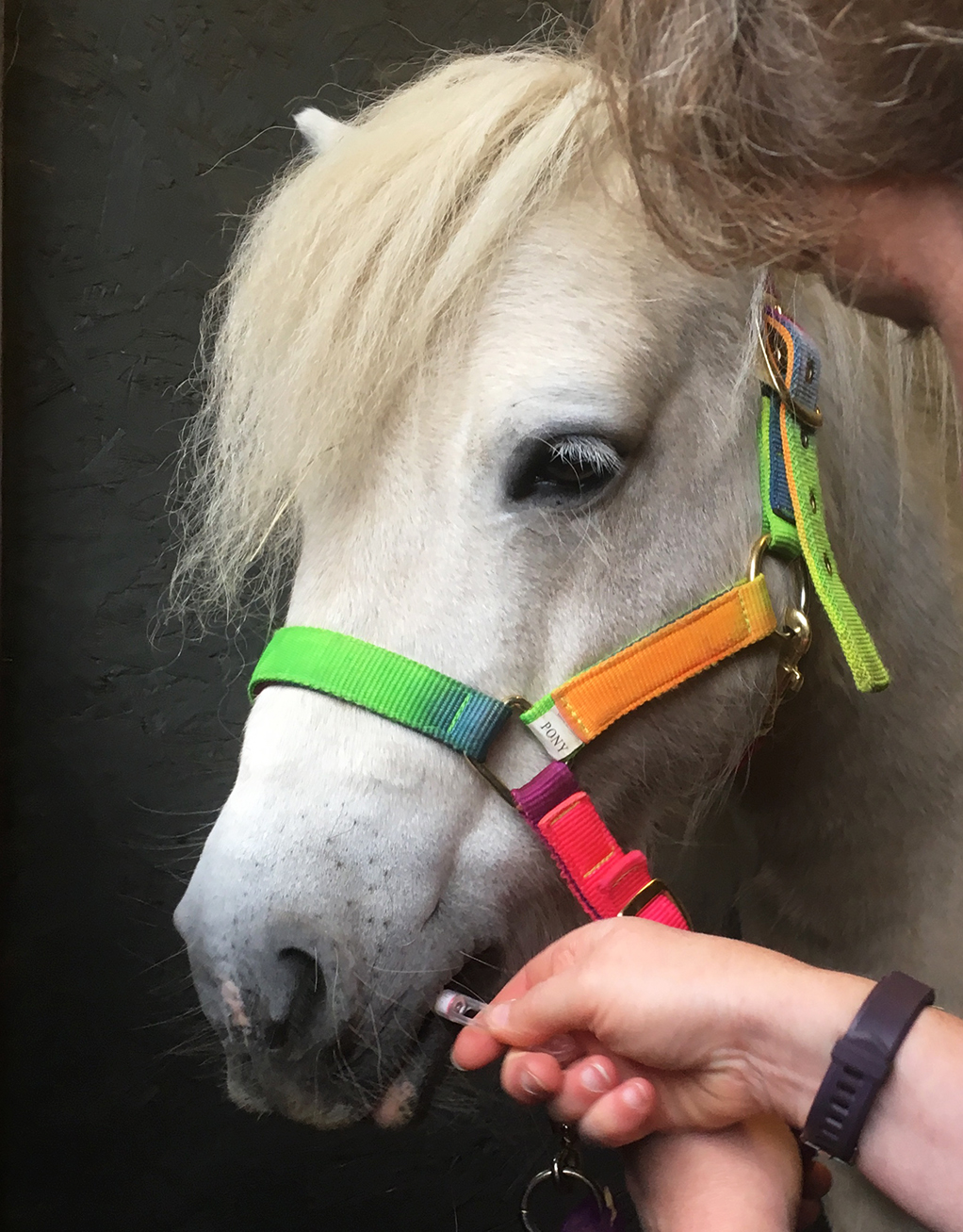
The EquiSal Tapeworm Saliva Test (Austin Davis Biologics) is being increasingly used in the UK to inform anti-tapeworm treatments. This test measures salivary IgG(T) to A perfoliata excretory/secretory antigens using a three-test format that accounts for specific saliva properties, including flow rate. Based on the level antibody measured, the test classifies results as “low”, “borderline” and “moderate/high”, with treatment advised for horses with borderline or moderate/high results. This test has been used in more than 200,000 occasions so far and, of these, only 1 in 3 test results indicates a requirement for treatment; therefore, reducing anti-tapeworm anthelmintic applications considerably25.
A further antibody test, the Small Redworm Blood Test (Austin Davis Biologics), has been available in the UK since 20194. This ELISA is based on the measurement of serum IgG(T) to cloned recombinant proteins that are specific to cyathostomins and have been shown to be expressed and detectable in all parasitic stages, including all encysted larval stages26,27. This test detects low cyathostomin burdens with high sensitivity, and is suitable for use in horses at low-infection risk and where owners continue to intend to apply an annual encysted larvicidal treatment that may be unnecessary.
When used according to the test guidelines, use of this diagnostic can result in considerable reductions in anthelmintic applications compared to a blanket treatment approach25.
Conclusions
Although some of the UK equine sector has moved away from the previously almost-ubiquitous application of interval worming treatments in horses, recent surveys show that some way is still left to go to achieve the level of uptake needed to address anthelmintic resistance.
The development of accessible new guidelines to support prescribers in applying evidence-based methods of control should improve the dialogue around the use of anthelmintics on yards and farms.
In the pipeline are veterinary-specific and cross-sector tools from BEVA and an initiative known as CANTER (Controlling ANTiparasitic resistance in Equines Responsibly – https://canterforhorses.org.uk).
It is hoped that the availability of these guidelines will help encourage horse owners to engage in control programmes that will rely less on the dwindling supply of effective anthelmintics.
References
- Nielsen MK (2022). Anthelmintic resistance in equine nematodes: current status and emerging trends, Int J Parasitol Drugs Drug Resist 20: 76-88.
- Nielsen MK (2023). Apparent treatment failure of praziquantel and pyrantel pamoate against anoplocephalid tapeworms, Int J Parasitol Drugs Drug Resist 22: 96-101.
- Lightbody KL et al (2016). Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses, Vet Clin Pathol 45(2): 335-346.
- Lightbody KL et al (2024). Validation of a serum ELISA test for cyathostomin infection in equines, Int J Parasitol 54(1): 23-32.
- Wood ELD et al (2013). Variation in fecal egg counts in horses managed for conservation purposes: individual egg shedding consistency, age effects and seasonal variation, Parasitology 140(1): 115-128.
- Drudge JH and Lyons ET (1966). Control of internal parasites of the horse, J Am Vet Med Assoc 148(4): 378-383.
- Relf VE et al (2013). Helminth egg excretion with regard to age, gender and management practices on UK Thoroughbred studs, Parasitology 140(5): 641-652.
- Uhlinger CA (1993). Uses of fecal egg count data in equine practice, Compend Contin Educ Practicing Vet 15(5): 742-748.
- Larsen ML et al (2011). Determination of ivermectin efficacy against cyathostomins and Parascaris equorum on horse farms using selective therapy, Vet J 188(1): 44-47.
- van Wyk JA (2001). Refugia – overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance, Onderstepoort J Vet Res 68(1): 55-67.
- Leathwick DM et al (2012). Managing anthelmintic resistance – use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin, Vet Parasitol 187(1-2): 285-294.
- Menzel M et al (2012). Implementation of selective anthelmintic treatment in an equine-practice in Upper Bavaria (Germany): 1st experiences, J Equine Vet Sci 32(10): S35-S36.
- Nielsen MK et al (2006). Strongyle egg shedding consistency in horses on farms using selective therapy in Denmark, Vet Parasitol 135(3-4): 333-335.
- Becher AM et al (2010). Selective anthelmintic therapy of horses in the Federal states of Bavaria (Germany) and Salzburg (Austria): an investigation into strongyle egg shedding consistency, Vet Parasitol 171(1-2): 116-122.
- Lester HE et al (2012). The spatial distribution of strongyle eggs in horse faeces, J Equine Vet Sci 32(10): S33-S34.
- Nielsen MK et al (2010). Effects of fecal collection and storage factors on strongylid egg counts in horses, Vet Parasitol 167(1): 55-61.
- Denwood MJ et al (2012). Quantifying the sources of variability in equine faecal egg counts: implications for improving the utility of the method, Vet Parasitol 188(1-2): 120-126.
- Lester HE and Matthews JB (2014). Faecal worm egg count analysis for targeting anthelmintic treatment in horses: points to consider, Equine Vet J 46(2): 139-145.
- Ministry of Agriculture, Fisheries and Food (1986). Manual of Veterinary Parasitological Laboratory Techniques, Her Majesty’s Stationery Office, London.
- Torgerson PR et al (2012). The contribution of simple random sampling to observed variations in faecal egg counts, Vet Parasitol 188(3-4): 397-401.
- Matthews JB et al (2023). The use of innovative diagnostics to inform sustainable control of equine helminth infections, Pathogens 12(10): 1,233.
- Gasser RB et al (2005). Anoplocephala perfoliata of horses, Parasitology 131(1): 1-13.
- Meana A et al (2005). Epidemiological studies on equine cestodes in central Spain: infection pattern and population dynamics, Vet Parasitol 130(3-4): 233–240.
- Proudman CJ and Trees AJ (1996). Correlation of antigen specific IgG and IgG(T) responses with Anoplocephala perfoliata infection intensity in the horse, Parasite Immunol 18(10): 499-506.
- Matthews J et al (2024). Latest developments in testing for equine helminths, In Pract 46(1): 34-41.
- McWilliam HEG et al (2010). Identification and characterisation of an immunodiagnostic marker for cyathostomin developing stage larvae, Int J Parasitol 40(3): 265-275.
- Tzelos T et al (2020). Characterisation of serum IgG(T) responses to potential diagnostic antigens for equine cyathostominosis, Int J Parasitol 50(4): 289-298.